Health Policy & Bioethics Consortia 2020-2021
PORTAL runs a monthly seminar series that convenes two experts from different fields or vantage points to discuss how various aspects of therapeutic development and use are affected by ethical norms, laws, and regulations. These are organized in conjunction with the HMS Center for Bioethics supported by the Oswald DeN. Cammann Fund at Harvard University.
You may join the discussion on Twitter before, during, or after each event by following @PORTAL_Research and using the hashtag #policyethx.
You may join the discussion on Twitter before, during, or after each event by following @PORTAL_Research and using the hashtag #policyethx.
Health Care for Incarcerated Populations
April 9, 2021 | 12:30 - 2:00 PM ET
Webinar via Zoom (Register HERE)
April 9, 2021 | 12:30 - 2:00 PM ET
Webinar via Zoom (Register HERE)
|
EXPERTS:
|
MODERATOR:
|
PAST EVENTS
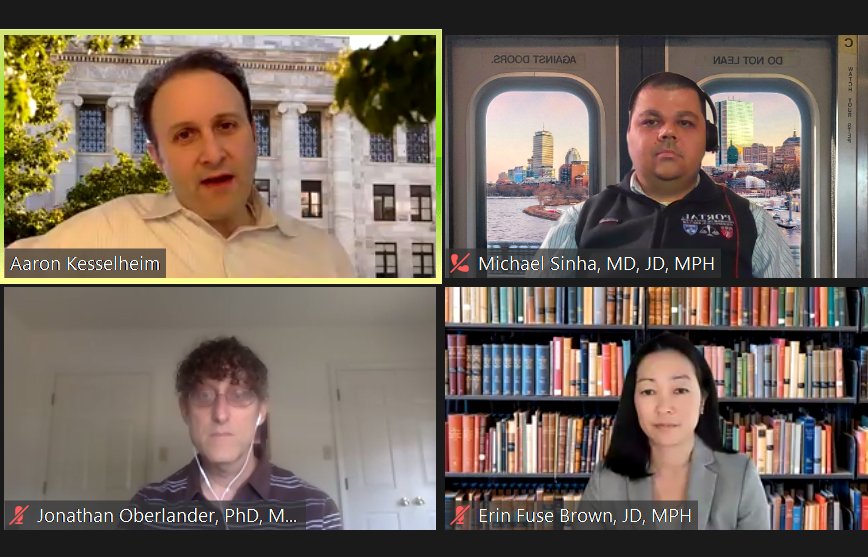
The ACA in the 2020s: Where Does U.S. National Health Coverage Go from Here?
Since its passage over a decade ago, the Affordable Care Act (ACA) has expanded health care coverage for Americans but also faced constant litigation and uncertainty about its future, particularly in the last four years. With a new political administration now in place, the panelists will discuss the current state of ACA litigation and federal and state efforts that could improve the ACA and health care coverage in the U.S.
March 12, 2021 | Webinar via Zoom
Since its passage over a decade ago, the Affordable Care Act (ACA) has expanded health care coverage for Americans but also faced constant litigation and uncertainty about its future, particularly in the last four years. With a new political administration now in place, the panelists will discuss the current state of ACA litigation and federal and state efforts that could improve the ACA and health care coverage in the U.S.
March 12, 2021 | Webinar via Zoom
|
EXPERTS:
Erin C. Fuse Brown, JD, MPH Cathy C. Henson Professor of Law Director, Center for Law, Health & Society Georgia State University College of Law Jonathan Oberlander, PhD, MPhil, MA Professor, Health Policy and Management Chair, Department of Social Medicine University of North Carolina at Chapel Hill MODERATOR: Michael S. Sinha, MD, JD, MPH Research Fellow, Harvard-MIT Center for Regulatory Science, Harvard Medical School Adjunct Faculty, Northeastern University School of Law |
Medical Stereotypes: Confronting Racism and Disparities in US Health Care
February 12, 2021 | Webinar via Zoom
Minority racial and ethnic groups in the US have long experienced disparities in access to health care and worse health outcomes, undermining broader social, political, and economic equality. One component of these disparities are various stereotypes that affect how minority patients are perceived and treated. This session will explore how medical discourse and the health system it supports can be altered to address these harms, and how legal changes can improve outcomes in the future.
February 12, 2021 | Webinar via Zoom
Minority racial and ethnic groups in the US have long experienced disparities in access to health care and worse health outcomes, undermining broader social, political, and economic equality. One component of these disparities are various stereotypes that affect how minority patients are perceived and treated. This session will explore how medical discourse and the health system it supports can be altered to address these harms, and how legal changes can improve outcomes in the future.
|
EXPERTS:
Evelynn Hammonds, PhD Chair, Department of the History of Science Barbara Gutmann Rosenkrantz Professor of the History of Science Professor of African American Studies Harvard University Craig Konnoth, JD, MPhil Associate Professor of Law Director of Health Law Certificate University of Colorado MODERATOR: Michelle Morse, MD, MPH Hospitalist, Brigham and Women's Hospital Assistant Professor, Harvard Medical School |
|
Access to Investigational Drugs and Stem Cell Treatments via Right to Try Laws: Legal and Ethical Considerations
December 11, 2020 | Webinar via Zoom
Access to investigational drugs has been a controversial topic during the COVID19 pandemic: what is the level of evidence required? How do we evaluate individual access requests, particularly from public figures such as outgoing Presidents? How does providing access change the ability to acquire fundamental information about these products? In May 2018, the Right to Try Act was signed into law, creating a new pathway for patients with life-threatening conditions to access investigational products prior to FDA approval. Prior to the federal law, more than half of states had passed their own versions of right to try. Two expert panelists will consider the scientific, legal, and ethical issues relating to access to investigational treatments and how Right to Try laws change the landscape for the future.
December 11, 2020 | Webinar via Zoom
Access to investigational drugs has been a controversial topic during the COVID19 pandemic: what is the level of evidence required? How do we evaluate individual access requests, particularly from public figures such as outgoing Presidents? How does providing access change the ability to acquire fundamental information about these products? In May 2018, the Right to Try Act was signed into law, creating a new pathway for patients with life-threatening conditions to access investigational products prior to FDA approval. Prior to the federal law, more than half of states had passed their own versions of right to try. Two expert panelists will consider the scientific, legal, and ethical issues relating to access to investigational treatments and how Right to Try laws change the landscape for the future.
|
|
EXPERTS:
George Q. Daley, MD, PhD Dean of the Faculty of Medicine Harvard Medical School Alison Bateman-House, MPH, PhD Assistant Professor, Department of Population Health NYU Grossman School of Medicine MODERATOR: Insoo Hyun, PhD Professor, Department for Bioethics, Case Western University School of Medicine Faculty Member, Center for Bioethics, Harvard Medical School |
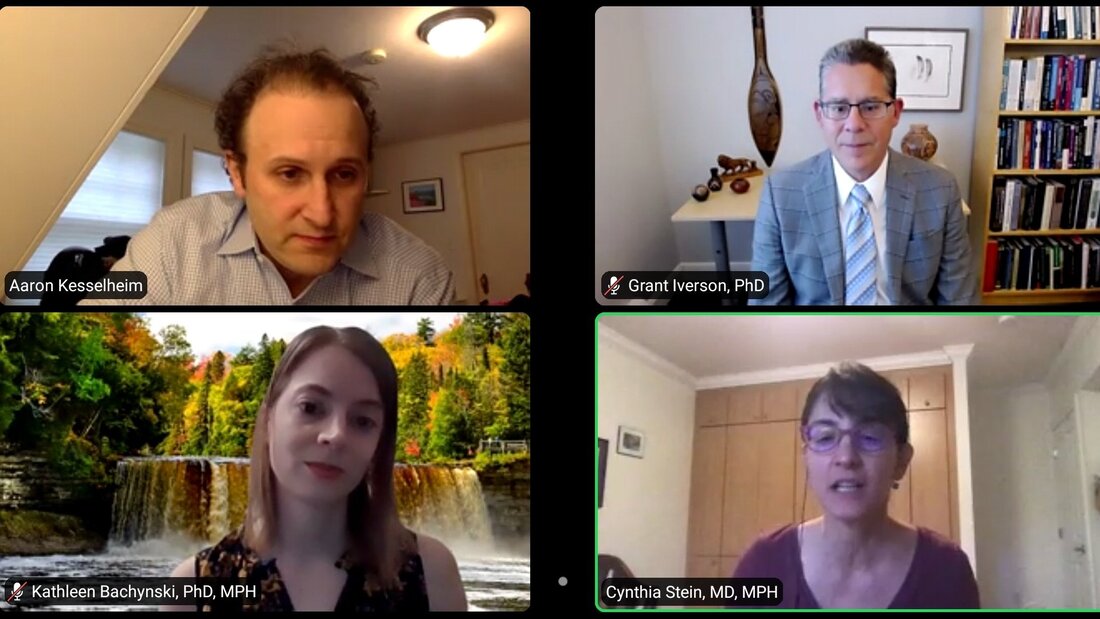
The NFL, Youth Sport, and Concussions
November 13, 2020 | Webinar via Zoom
Potential links between contact sports and chronic traumatic encephalopathy or other long-lasting neurological effects have received renewed public interest with high-profile lawsuits and debate about the dangers of sport. There are challenges with the evidence for drawing firm conclusions, making these debates especially contentious. The concerns about sport and head injury, especially for young players, are not new. Despite recognition of the dangers inherent in contact sports, there is a history of avoiding addressing the problem head-on. American culture surrounding football, lauded for its rough style of play, also influences our understandings of risk, injury, and changes to the sport.
November 13, 2020 | Webinar via Zoom
Potential links between contact sports and chronic traumatic encephalopathy or other long-lasting neurological effects have received renewed public interest with high-profile lawsuits and debate about the dangers of sport. There are challenges with the evidence for drawing firm conclusions, making these debates especially contentious. The concerns about sport and head injury, especially for young players, are not new. Despite recognition of the dangers inherent in contact sports, there is a history of avoiding addressing the problem head-on. American culture surrounding football, lauded for its rough style of play, also influences our understandings of risk, injury, and changes to the sport.
|
EXPERTS:
Kathleen Bachynski, PhD, MPH Assistant Professor Muhlenberg College Grant Iverson, PhD Professor, Dept of Physical Medicine and Rehabilitation Harvard Medical School Director, Sports Concussion Program MassGeneral Hospital for Children MODERATOR: Cynthia Stein, MD, MPH Attending Physician, Sports Medicine Division Dept of Orthopedic Surgery Director, Medical Sports Medicine Fellowship Program Boston Children's Hospital |
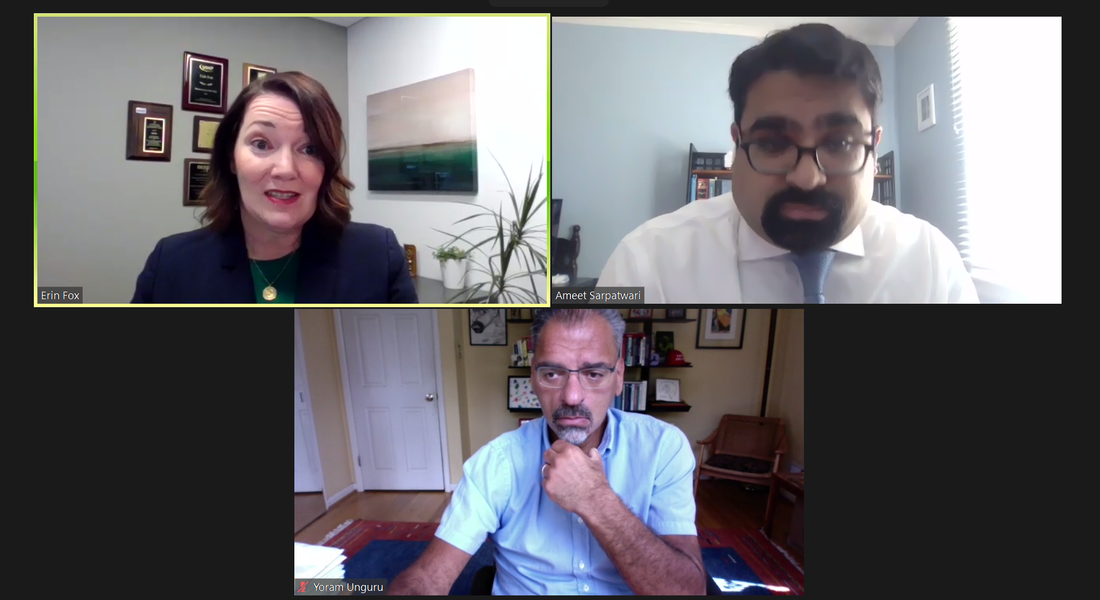
Drug Shortages: Managing Prioritization & Improving the Supply Chain
October 9, 2020 | Webinar via Zoom
Drug shortages, when the supply of a drug is not adequate to meet current or near-term future demand, have become increasingly common in the U.S. These shortages occur for a variety of reasons, including disruptions to supply chains, manufacturing stoppages, and economic incentives. Drug shortages have real effects on patients and result in medication errors, delayed life-saving treatment, and worse patient outcomes. We will examine what policies could change the current situation and ensure the consistent supply of drugs. Shortages also raise important questions to consider about what clinicians should do during a shortage and how to make decisions about which patients to prioritize for treatment with a scarce resource.
October 9, 2020 | Webinar via Zoom
Drug shortages, when the supply of a drug is not adequate to meet current or near-term future demand, have become increasingly common in the U.S. These shortages occur for a variety of reasons, including disruptions to supply chains, manufacturing stoppages, and economic incentives. Drug shortages have real effects on patients and result in medication errors, delayed life-saving treatment, and worse patient outcomes. We will examine what policies could change the current situation and ensure the consistent supply of drugs. Shortages also raise important questions to consider about what clinicians should do during a shortage and how to make decisions about which patients to prioritize for treatment with a scarce resource.
|
EXPERTS:
Erin Fox, PharmD, BCPS, FASHP Senior Director, Drug Information and Support Services, University of Utah Yoram Unguru, MD, MS, MA Assistant Professor of Medicine Johns Hopkins University MODERATOR: Ameet Sarpatwari, PhD, JD Assistant Professor of Medicine Harvard Medical School and Brigham and Women's Hospital Assistant Director, PORTAL |
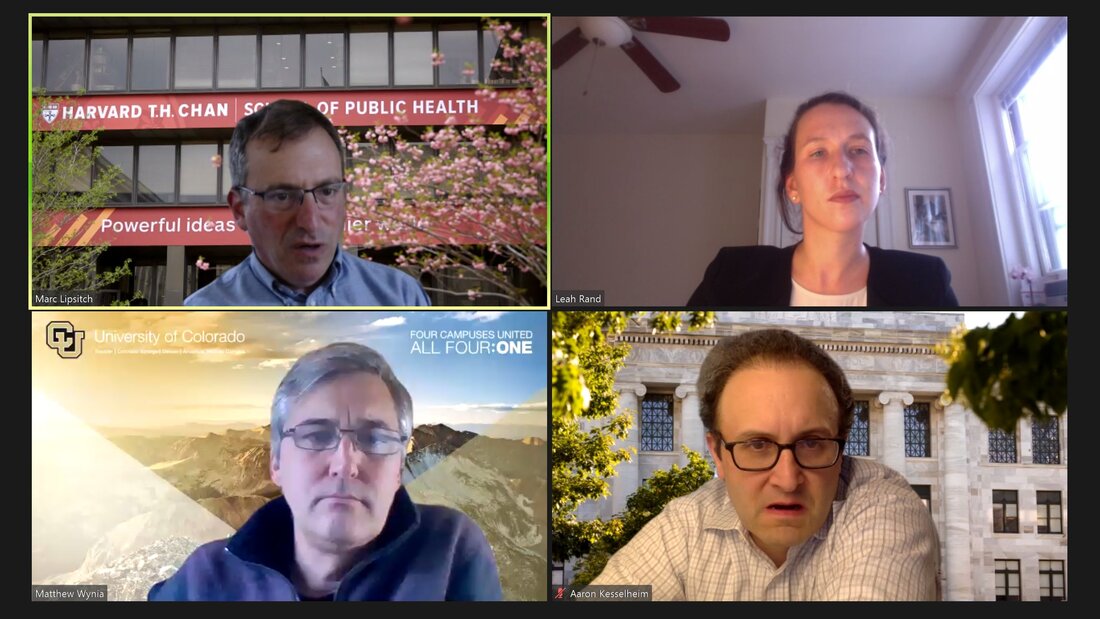
COVID-19, Public Health Ethics and Policy for Pandemics
September 11, 2020 | Webinar via Zoom
Since the SARS-CoV-2 pandemic began, scientific recommendations about the best way to reduce its impact have met wide-ranging resistance in the U.S. With two experts in infectious disease epidemiology and ethics, we will review how scientific evidence is collected in a rapidly changing public health environment, and how to translate that evidence into actionable recommendations. We will also discuss the sources of controversy that have arisen as the pandemic has evolved, including balancing individual and public health interests and how to manage different types of uncertainty.
September 11, 2020 | Webinar via Zoom
Since the SARS-CoV-2 pandemic began, scientific recommendations about the best way to reduce its impact have met wide-ranging resistance in the U.S. With two experts in infectious disease epidemiology and ethics, we will review how scientific evidence is collected in a rapidly changing public health environment, and how to translate that evidence into actionable recommendations. We will also discuss the sources of controversy that have arisen as the pandemic has evolved, including balancing individual and public health interests and how to manage different types of uncertainty.
|
EXPERTS:
Marc Lipsitch, DPhil Professor of Epidemiology Director, Center for Communicable Disease Dynamics Department of Epidemiology Harvard T.H. Chan School of Public Health Matthew Wynia, MD, MPH Director, Center for Bioethics and Humanities University of Colorado Anschutz Medical Campus MODERATOR: Leah Rand, PhD, MA Research Fellow, PORTAL |
|
Program On Regulation, Therapeutics And Law (PORTAL)
Division of Pharmacoepidemiology and Pharmacoeconomics 1620 Tremont Street, Suite 3030 Boston, MA 02120 |